Background: Current treatments for MM consist of multidrug regimens combining a variety of drug classes including IMiDs, proteasome inhibitors (PIs), monoclonal antibodies (mAbs), alkylators, XP01 inhibitors, histone deacetylase inhibitors, and corticosteroids. Despite advances in therapy, outcomes remain poor for pts with RRMM. Melphalan flufenamide (melflufen) is a first-in-class peptide-drug conjugate (PDC) that targets aminopeptidases and rapidly releases alkylating agents into tumor cells. In the pivotal, phase 2 HORIZON study (NCT02963493), melflufen plus dex showed meaningful efficacy and a safety profile characterized primarily by clinically manageable hematologic adverse events (AEs) in heavily pretreated and poor-risk pts with RRMM (Richardson et al. EHA 2020. Abs. EP945). Preclinical data show that melflufen is 50-fold more potent than melphalan against MM cells due to an increased intracellular alkylator concentration (Wickstrom et al. Oncotarget. 2017;8:666641). Melflufen has a mechanism of action distinct from alkylators, with cytotoxicity independent of p53 activity (Slipicevic et al. AACR 2020. Abs. 1843). In this analysis, the clinical activity of melflufen is examined in a subset of pts exposed to prior alkylator therapy.
Methods: Pts with RRMM had received ≥2 prior lines, including an IMiD and PI, and were refractory to pomalidomide and/or an anti-CD38 mAb. Pts received melflufen 40 mg on d1 of each 28-d cycle plus dex 40 mg/wk until progressive disease or unacceptable toxicity. The primary endpoint was overall response rate (ORR; ≥ partial response [PR]; assessed by the investigator per International Myeloma Working Group criteria). Secondary endpoints included duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety. Refractoriness was defined as disease that failed to achieve a minimal response or progressed while on primary or salvage therapy or progressed within 60 d of last therapy.
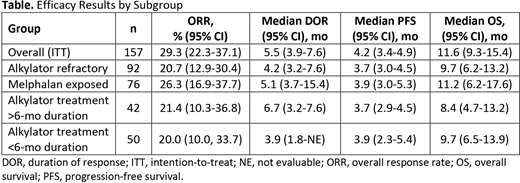
Results: Of 157 pts included (data cutoff date, Jan 14, 2020), 138 (88%) had been exposed to and 92 (59%) were refractory to prior alkylator therapy. Baseline characteristics for pts refractory to prior alkylators were generally consistent with those of the overall population apart from more advanced International Staging System score (ISS 3, 34% and 25%), number of prior lines of therapy (6 and 5), and triple-class-refractoriness (83% and 76%). As in the overall population, a high proportion of pts refractory to alkylators had high-risk cytogenetics (40%) and extramedullary disease (36%), representing a difficult-to-treat group. Overall, 60% of pts refractory to alkylators had been exposed to (median time since last exposure, 1.3 y) and 27% were refractory to an alkylator in ≥1 line, and 22% were refractory to an alkylator in the last line. The ORR (95% CI) was 20.7% (12.9-30.4) in pts refractory to prior alkylators and 29.3% overall (Table). Among responding pts refractory to prior alkylators (n=19), 1 achieved a stringent complete response, 6 a very good PR, and 12 a PR. Median DOR (95% CI) was 4.2 mo (3.2-7.6) in pts refractory to prior alkylators and 5.5 mo (3.9-7.6) overall. Median PFS (95% CI) was 3.7 mo (3.0-4.5) and 4.2 mo (3.4-4.9), respectively, and OS was 9.7 mo (6.2-13.2) and 11.6 mo (9.3-15.4), respectively, in pts refractory to prior alkylators and overall. ORR (95% CI) was 20.0% (10.0-33.7) and 21.4% (10.3-36.8) in pts who had received alkylator treatment for <6 mo and >6 mo, respectively; ORR in pts exposed to melphalan was 26.3% (95% CI, 16.9-37.7).
The safety profile of melflufen plus dex in pts refractory to prior alkylators was consistent with that in the overall population. In pts refractory to prior alkylators and the overall population, grade 3/4 AEs were reported in 91% and 89% of pts, respectively most commonly thrombocytopenia (61% and 57%), neutropenia (57% and 53%), and anemia (43% and 43%). The most common nonhematologic AE was pneumonia (11% and 10%). Serious AEs occurred in 55% of pts refractory to prior alkylators and 49% of pts overall; most commonly pneumonia (9% and 9%) and febrile neutropenia (7% and 5%).
Conclusion: Melflufen has a distinct mechanism of action from that of melphalan and other alkylators and in combination with dex showed efficacy in pts with disease refractory to prior alkylator therapy with a manageable safety profile. These results are consistent with those of previous preclinical and clinical studies.
Rodríguez-Otero:Janssen, BMS: Other: Travel, accommodations, expenses; Celgene-BMS: Consultancy, Honoraria; Mundipharma: Research Funding; BMS, Janssen, Amgen: Honoraria; Janssen, BMS, AbbVie, Sanofi, GSK, Oncopeptides, Kite, Amgen: Consultancy, Honoraria. Mateos:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Oriol:Janssen: Consultancy; Sanofi: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees. Larocca:Amgen: Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Blade Creixenti:Janssen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Cavo:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Honoraria; GlaxoSmithKline: Honoraria, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Leleu:AbbVie: Honoraria; Novartis: Honoraria; Amgen: Honoraria; GSK: Honoraria; Sanofi: Honoraria; Oncopeptide: Honoraria; Karyopharm: Honoraria; Incyte: Honoraria; Merck: Honoraria; BMS-celgene: Honoraria; Janssen: Honoraria; Carsgen: Honoraria. Nadeem:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Amgen: Membership on an entity's Board of Directors or advisory committees; Adaptive: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES. Hassoun:Novartis: Consultancy; Celgene: Research Funding; Takeda: Research Funding. Touzeau:Amgen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; GlaxoSmithKline: Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Sanofi: Honoraria, Research Funding. Amor:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Maisel:Takeda: Honoraria, Speakers Bureau; Texas Oncology: Current Employment; Amgen: Honoraria, Speakers Bureau; Texas Oncology: Current Employment; Celgene: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Kite: Honoraria, Speakers Bureau; Celgene: Honoraria, Speakers Bureau; Incyte: Honoraria, Speakers Bureau; Karyopharm: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Kite: Honoraria, Speakers Bureau; Incyte: Honoraria, Speakers Bureau; Karyopharm: Honoraria, Speakers Bureau. Mazumder:Takeda: Honoraria, Speakers Bureau; The Oncology Institute: Current Employment; Celgene: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Raptis:INTEGRA: Consultancy, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; UPMC: Current Employment. Puig:JANSSEN: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding; TAKEDA: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding; AMGEN: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding; CELGENE: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding, Speakers Bureau; BRISTOL-MYERS SQUIBB: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding, Speakers Bureau; THE BINDING SITE: Consultancy, Honoraria. Thuresson:Statisticon: Current Employment; Oncopeptides: Consultancy, Current equity holder in publicly-traded company. Harmenberg:Ultupharma AB: Current equity holder in private company; Medivir AB: Current equity holder in publicly-traded company; Oncopeptides AB: Consultancy, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Other: TRAVEL, ACCOMMODATIONS, EXPENSES. Harlin:Oncopeptides AB: Consultancy, Current equity holder in publicly-traded company. Richardson:Celgene/BMS, Oncopeptides, Takeda, Karyopharm: Research Funding.
This is a phase 2 investigational study of melflufen in RRMM.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal